Article Text
Abstract
Background: Although many children with asthma may have a remission as they grow and other children who did not have asthma may develop asthma in adult life, knowledge about the factors that influence the onset and prognosis of asthma during adolescence and young adulthood is very limited.
Methods: A cohort of 8–10 year old children (n=718) living in Belmont, New South Wales, Australia were surveyed six times at 2 yearly intervals from 1982 to 1992, and then again 5 years later in 1997. From this cohort, 498 subjects had between three and seven assessments and were included in the analysis. Atopy, airway hyperresponsiveness (AHR), and wheeze in the last 12 months were measured at each survey. Late onset, remission, and persistence were defined based on characteristics at the initial survey and the changes in characteristics at the follow up surveys.
Results: The proportion of subjects with late onset atopy (13.7%) and wheeze (12.4%) was greater than the proportion with remission of atopy (3.2%) and wheeze (5.6%). Having atopy at age 8–12 years (OR 2.8, 95% CI 1.5 to 5.1) and having a parental history of asthma (OR 2.0, 95% CI 1.02 to 4.13) were significant risk factors for the onset of wheeze. Having AHR at age 8–12 years was a significant risk factor for the persistence of wheeze (OR 4.3, 95% CI 1.3 to 15.0). Female sex (OR 1.9, 95% CI 1.01 to 3.60) was a significant risk factor for late onset AHR whereas male sex (OR 1.9, 95% CI 1.1 to 2.8) was a significant risk factor for late onset atopy.
Conclusions: The onset of AHR is uncommon during adolescence, but the risk of acquiring atopy and recent wheeze for the first time continues during this period. Atopy, particularly present at the age of 8–10 years, predicts the subsequent onset of wheeze.
- asthma
- atopy
- wheeze
- risk factors
- adolescence
Statistics from Altmetric.com
Asthma is a long term disease that may initially manifest during childhood or adult life. It is recognised that many children with asthma have remission of the disease as they grow and other children, who do not have asthma, may develop asthma in adult life. However, we have very limited knowledge about the factors that influence the onset and prognosis of asthma during adolescence and young adulthood.1 Results from cross sectional studies have shown that there are associations between atopy, respiratory symptoms, and airway hyperresponsiveness,2–,5 but little is known about how any of these factors influences the development of the other characteristics.
Although genetic factors are associated with atopy and asthma, the inheritance of atopy may be different from the inheritance of asthma6 and parental histories of asthma and allergic disease may have different influences on the development of atopy and asthma in children.7 In addition, sex difference plays a role in the development of asthma. There is a male predominance during childhood, but this is reversed after puberty.8 However, we do not know whether the female predominance in asthma after puberty is attributable to a higher rate of onset of asthma in females during adolescence or to a lower remission rate.
Factors that influence the onset and the progression of atopy and asthma during adolescence and young adulthood can only be investigated in longitudinal population studies. We have used data from a longitudinal population study to examine the onset and remission of atopy and asthma during adolescence and to evaluate potential risk factors.
METHODS
Subjects
In 1982 a large random sample of third and fourth grade schoolchildren aged 8–10 years living in the coastal town of Belmont, New South Wales, Australia was studied. The study methods have been described previously in detail.9 A total of 718 children participated in the initial survey in 1982, representing a response rate of 87%. From 1984 to 1992 five follow up surveys were conducted at 2 year intervals. Between 1997 and 1999 the seventh survey was conducted. At each follow up survey every effort was made to contact each subject initially enrolled. The age ranges and the number of subjects who participated in each survey are shown in table 1⇓. All surveys were undertaken in the winter months. Of the cohort of 718 subjects, 498 (69%) took part in a further two or more surveys between 1986 (survey 3) and 1997 (survey 7) and composed the selected group for this analysis.
Age range and number of subjects who participated in each follow up survey
Questionnaires
In the studies between 1982 and 1988 (when the subjects were under 16 years of age), a questionnaire was completed by a parent of each study subject. From 1990 each subject completed a self-administered questionnaire. Questions about symptoms (wheeze, wheeze after exercise), diagnosed asthma, and medication use were asked. Subjects who reported wheeze during the previous year of the study were classified as having “recent wheeze”.
Lung function and airway hyperresponsiveness
Lung function of each subject was measured at each survey using a Vitalograph dry spirometer from 1982 to 1990 and a dry rolling seal spirometer linked to a personal computer in 1992 and 1997–9, performed with the subject standing and without a nose clip. Forced expiratory manoeuvres were repeated until two readings of forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) within 100 ml were obtained, the largest of which was used in analyses.
A bronchial challenge test with histamine was administered to all subjects with baseline FEV1 ≥60% predicted using the rapid method.10 After measurement of baseline lung function, the FEV1 was recorded again after inhalation of saline. Histamine diphosphate was then administered using glass hand held nebulisers from 1982 to 1986 and using DeVilbiss hand held nebulisers in surveys on and after 1988 (DeVilbiss Co, Heston, UK) in doubling doses ranging from 0.03 to 3.92 μmol. The test was stopped if the FEV1 fell by 20% or more or if all histamine dose steps to 3.92 μmol had been administered. Salbutamol aerosol was given to aid recovery when necessary. For subjects who had a fall in FEV1 of 20% or greater, the dose of histamine that caused a 20% fall (PD20FEV1) was calculated. Subjects with a PD20FEV1 of ≤3.92 μmol were classified as having airway hyperresponsiveness (AHR). Subjects who had used a β agonist within 6 hours of presenting were asked to withhold medication before returning for later testing.
Skin prick tests
Sensitisation to common allergens was measured by reactions to skin prick tests administered to the forearm.11 The eight allergens tested were: house dust, house dust mite (Dermatophagoides pteronyssinus and D farinae), cat dander, ryegrass, plantain, Alternaria alternata (tenuis), and cockroach. Histamine and glycerol were used as positive and negative controls. Subjects with a negative histamine or a positive glycerol test were retested. After 15 minutes the wheal size was recorded as the long axis and its perpendicular; mean wheal size was used in analyses. A reaction was regarded as positive if the wheal size was 3 mm or greater. Subjects were considered atopic if they had a positive reaction to any of the allergens tested.
Definitions
Late onset and remission of wheeze, AHR, and atopy were defined based on the condition of the subject at the first survey and in follow up surveys. Subjects who did not have the characteristic at any survey were defined as “never” having the characteristic. Subjects who did not have the characteristic at surveys 1 and 2 (if they participated) but who did have it at one or more subsequent surveys were defined as having “late onset” of the characteristic if it was present in two or more consecutive surveys in which they participated, and otherwise were defined as having the characteristic “intermittently” if it was not present in two consecutive surveys. Subjects who had the characteristic at one of the first two surveys and who did not have it at two subsequent consecutive surveys in which they participated were defined as having had a remission. Other subjects who had a characteristic at one of the first two surveys but did not experience a remission were defined as having persistence of the characteristic.
To evaluate the relation between time at which atopy was acquired and late onset of wheeze, the cohort was further divided into four groups: never atopic; atopy started after age 12; atopy started between ages 10 and 12; and atopy started before age 10.
Statistical methods
Data were analysed using the statistical package SAS (SAS Institute Inc, Cary, NC, USA). Prevalence and mean values are presented with their 95% confidence intervals. Chi-square tests were used to determine the significance of differences in prevalence between samples. Logistic regression was used to calculate adjusted odds ratios of the risk factors measured at age 8–12 years for late onset and persistent of recent wheeze, AHR, and atopy.
RESULTS
Table 2⇓ shows baseline characteristics of the selected group (n=498) and the remaining group (n=220). There were no significant differences between the two groups in the prevalence of atopy, recent wheeze, AHR, and baseline lung function at survey 1, suggesting that the selected group was representative of the initial cohort.
Baseline characteristics at survey 1 of the selected group in the present data analysis and the remaining group
Onset and remission rate of recent wheeze, AHR, and atopy
Table 3⇓ shows the rates of onset and remission of recent wheeze, AHR, and atopy. Of the 498 subjects 301 (60.4%) never had recent wheeze during the study period. The 14% of the cohort who had recent wheeze between the ages of 8 and 12 years (either in survey 1 or in survey 2) comprised 42 subjects (8.4%) who had persistent wheeze and 28 (5.6%) who had wheeze that remitted during the follow up period. The proportion of late onset wheeze (12.4%) was higher than the rate of remission (5.6%), and the prevalence of recent wheeze in this cohort increased by 6% between 1982 and 1992.
Prognosis and development of recent wheeze, airway hyperresponsiveness (AHR), and atopy in the study sample (n=498)
In contrast to the findings for recent wheeze, the rate of remission of AHR (7.2%) was greater than the rate of late onset AHR (1.8%). However, a substantial number of subjects (7.8%) who did not have AHR at age 8–12 years developed AHR intermittently during the follow up period. Persistent AHR (4.4%) was less common than persistent recent wheeze (8.4%).
Fifty six percent of the cohort was identified as having atopy at one or more surveys during the study period. Persistent atopy (28%) was more common than atopy that remitted (3.2%). The proportion of subjects with late onset atopy (13.7%) was also higher than the proportion of subjects whose atopy remitted. Because there was a difference between the late onset rate and the remission rate, the prevalence of atopy increased by 10% from 1982 to 1992.
Risk factors for late onset and persistent wheeze
The presence of atopy at age 8–12 years and having either parent with asthma were independent risk factors for late onset wheeze (fig 1⇓). Among subjects who did not wheeze at age 8–12 years, those who had atopy at this age were 2.8 times (95% CI 1.5 to 5.1) more likely to have late onset wheeze than non-atopic subjects. Subjects with a parental history of asthma were 2.1 times (95% CI 1.02 to 4.13) more likely to have late onset wheeze than the other children after adjusting for the subject's atopic status. Having AHR at 8–12 years was not a significant risk factor for late onset wheeze after atopy at 8–12 years was taken into account. Being female was associated with a trend towards a higher risk of late onset wheeze after adjusting for atopy and parental history of asthma, but this was not statistically significant (adjusted OR 1.7, 95% CI 0.95 to 2.97). Among children who had recent wheeze at age 8–12 years, having AHR at that age range was a significant risk factor for persistence of recent wheeze during the follow up period (fig 1⇓). The presence of atopy at 8–12 years, sex, and a parental history of asthma were not independent risk factors for persistence of recent wheeze.
Risk factors for late onset (•) and persistence of recent wheeze (▪).
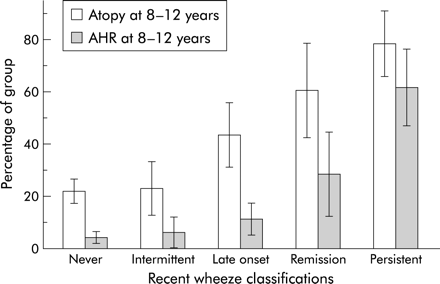
Figure 2⇓ shows the prevalence of atopy and AHR at age 8–12 years in subjects classified by recent wheeze history. To evaluate the relative importance of atopy and AHR at age 8–12 years on the future development of recent wheeze, the prevalence of atopy and AHR present at 8–12 years in the group who had late onset wheeze and the group who never had recent wheeze was compared and was significantly higher in the group with late onset wheeze (45% v 22%, p<0.01). However, the difference in prevalence of AHR at age 8–12 years was not statistically significant between the two groups (11.2% v 4.4%, p=0.1).
Proportion of atopy and airway hyperresponsiveness (AHR) at age 8–12 years among subgroups of recent wheeze classifications.
To assess the relative importance of atopy and AHR at age 8–12 years on persistent wheeze the prevalence of atopy and AHR at age 8–12 years in the group whose recent wheeze persisted and the group whose recent wheeze remitted was compared (fig 2⇑). The prevalence of AHR at age 8–12 years was significantly higher in subjects with persistent recent wheeze (60%) than in the group whose recent wheeze remitted (29%, p<0.01). However, the proportions with atopy at age 8–12 years were not significantly different between the two groups (78.6% v 60.7%, p=0.12).
Relation between time of acquiring atopy and late onset wheeze
Table 4⇓ shows the prevalence of late onset wheeze in each subgroup classified by the time when atopy was acquired (see Methods for definitions). Of the 429 subjects who did not have recent wheeze at the initial survey, there was a significantly increased trend in prevalence of late onset wheeze with an earlier time of atopy acquisition (p value for trend <0.001). Subjects who had atopy before the age of 10 were 3.9 times (95% CI 1.9 to 8.1) more likely to have late onset wheeze than non-atopic subjects.
Prevalence of late onset wheeze in 429 subjects divided into subgroups according to the age at which atopy was acquired
Risk factors for late onset AHR and atopy
To evaluate potential risk factors for development of AHR we combined the late onset AHR group and intermittent AHR group and compared them with the group with no AHR. Having atopy at age 8–12 years and being female were independent risk factors for development of AHR (late onset or intermittent). Subjects who had atopy at 8–12 years of age were 2.9 times (95% CI 1.5 to 5.4) more likely to develop AHR and girls were 1.9 times (95% CI 1.01 to 3.60) more likely than boys to develop AHR (fig 3⇓). However, having recent wheeze at age 8–12 years and a parental history of asthma were not risk factors for the subsequent development of AHR.
Risk factors for development (late onset and intermittent) of airway hyperresponsiveness (AHR).
In contrast to risk factors for development of AHR, being male was a risk factor for late onset atopy. Boys were 1.9 times (95% CI 1.1 to 2.8) more likely to have late onset atopy than girls (fig 4⇓), but a parental history of asthma and a parental history of other allergic diseases were not risk factors for late onset atopy.
Risk factors for late onset atopy.
DISCUSSION
During adolescence and young adulthood the onset rates of atopy and recent wheeze are higher than the remission rates. In fact, it was rare for subjects who were atopic at 8–12 years of age to become non-atopic during the subsequent period of the study. Among those who were not atopic at age 8–12 years, boys were more likely than girls to subsequently acquire atopy. The presence of atopy, particularly when present at 8–10 years of age, and a positive family history of asthma independently predicted the subsequent onset of wheeze. The onset of AHR after 12 years of age was relatively rare in this cohort, but it was more likely to occur in those who had been atopic at an early age. Independent of atopic status, girls were more likely than boys to acquire AHR during adolescence.
The strength of this study is that it was a population based cohort study with a wide range of subjective and objective outcome measures of allergy and asthma. The cohort was selected randomly from a general population of schoolchildren and the subjects who participated in the follow up studies did not differ significantly in baseline characteristics from those who were lost to follow up. Reliable methods of data collection were used through the whole study period and the length of follow up of the cohort was more than 15 years, with equivalent measurements made every 2 years in the first 10 years and then finally 5 years later. Information on symptoms derived from questionnaires was complemented by objective measures—that is, AHR measured by challenge test and atopy identified by skin prick tests. This comprehensive range of outcome measures has allowed us to define more accurately late onset and remission of asthma and atopy.
The two possible sources of measurement error in this study were the fact that questionnaires were completed by parents in the first four surveys and were self-completed in the last three surveys, and that the spirometers used for histamine challenge tests were changed during the course of the study. However, any error arising from these factors would almost certainly not be systematically related to the association between the exposures and outcomes of interest in this study, and hence would not be a source of systematic bias. Another limitation of the study is the broad confidence intervals around the effect estimates for persistent asthma and the consequent risk of a type II error. For example, with 70 subjects (14% of the cohort) who had recent wheeze at enrolment, the power to detect an odds ratio of 2.0 for persistent wheeze was 65%. This arises because, in a general population study, the number of subjects with asthma when the cohort was enrolled was relatively small.
Atopy acquired in childhood is associated with the onset of wheeze and AHR during adolescence and young adulthood. This finding is consistent with the observation in a previous study that atopy, defined as a positive skin prick test, was a significant risk factor for new diagnosis of asthma during the second decade of life.12 It has been well established, on the basis of cross sectional observations, that the presence of asthma is strongly linked to the presence of atopy in children and adults.13,14 However, the finding of this study expands our understanding by showing that the association between asthma and atopy extends beyond disease acquired during childhood. Atopic children who have not acquired wheeze and/or AHR by the age of 12 years are still at increased risk of developing these characteristics of asthma during subsequent years.
We found that the risk of developing asthma was related to the age at onset of atopy. Subjects who acquired atopy earlier in life have a higher risk of developing respiratory symptoms than subjects who acquired atopy later. There are several possible interpretations of this finding. One possibility is that there is a time delay between the manifestation of atopy, in the form of positive skin prick tests, and the expression of atopic disease such as asthma. If this were the case, then it would be expected that those with late onset atopy in this study would have a high rate of asthma during later follow up of this cohort. Alternatively, it is possible that early onset of atopy is required to initiate the airway changes which underlie the expression of asthma.
Consistent with the findings of other cohort studies,15,16 we found that the co-existence of AHR was a significant risk factor for persistence of wheeze. However, after the presence of AHR was taken into account, the presence of atopy was not an additional risk factor for the persistence of wheeze. We have previously shown that the co-existence of AHR and recent wheeze identifies a population with identifiable asthma morbidity, and have proposed that a combination of these attributes should be used as an epidemiological definition of asthma.17 The finding of this study, that children with asthma (defined in this way) are more likely to have persistent symptoms than other children with wheeze, lends longitudinal support to the validity of this epidemiological definition of asthma.
In the context of cross sectional and early childhood studies that show atopy to be a heritable characteristic,18,19 our finding that the onset of atopy after the age of 12 is not related to inherited factors suggests that the expression of atopy resulting from inherited factors is manifest before the age of 12. On the other hand, we found that parental history of asthma is a significant risk factor for the development of recent wheeze between the ages of 13 and 18 years in this cohort. This supports observations from other cohort studies. In a study of 1494 adults aged between 29 and 32 years, the risk of having asthma in adult life, defined as an attack of asthma in the previous 12 months, was 1.7 times higher (95% CI 1.2 to 2.5) if the mother or father had asthma.20 These findings show that the risk for asthma derived from inherited characteristics may not be manifest until adolescence or adult life, while inheritance of atopy is usually manifest earlier in childhood.
We found that a parental history of asthma did not have a significant negative effect on the prognosis of wheeze. Symptomatic children with either paternal or maternal asthma had a similar rate of remission of their symptoms to that of other children. Fewer long term studies have considered the influence of family history on the prognosis of childhood asthma. Our results suggest that environmental factors are probably more important than genetic factors in determining the prognosis of asthma symptoms and, hence, secondary prevention interventions directed at key environmental targets such as house dust mite allergen exposure21 may be beneficial.
Asthma and atopy are commonly acquired during adolescence and young adulthood. We found that more than 12% of the cohort developed asthma symptoms after the age of 12 years and that more than 13% developed atopy after this age. A previous study has found that the incidence of wheezing illness had an increased trend with age between 10 and 33 years.22 In our study new onset of wheeze accounted for more than half the prevalent cases after 12 years of age (12% late onset wheeze v 8% persistent wheeze) which suggests that the prevalence of asthma symptoms can increase with age during adolescence and young adulthood.
Interpretation of age related trends in cohort studies may be complicated by the effect of changing period effects during the course of the study. For example, if environmental factors that increase the expression of asthma were operating during the period 1982–97, we would have found an age related increase in the prevalence of asthma. In contrast, the interpretation of age related trends in cross sectional studies may be complicated by the effect of changing cohort effects during the time over which the study population was born. There is no perfect solution to this problem.23,24 However, examination of the current cohort study in the context of contemporary cross sectional studies25 suggests that there was a secular trend towards increasing asthma prevalence during the period of this study. Hence, at least part of the apparent age related increase in prevalence—that is, the onset of wheeze and atopy—may be attributable to period effects rather than true age effects.
Risk factors for the development of asthma and atopy have to be considered in the context of risk factors that have been assessed in cross sectional studies. In cross sectional studies, atopy and a family history of asthma are risk factors for wheeze and AHR during childhood.4,7 Taken in conjunction with the results of this study, we conclude that atopy that is evident by age 8–10 years and the genetic factors predict the presence or later acquisition of wheeze and/or AHR. Differences between the cross sectional association between sex and asthma and sex as a risk for subsequent development of asthma indicates that the sex related risk is age dependent. Boys are more at risk for asthma onset in early childhood while girls are more at risk in adolescence. On the other hand, male sex is associated with a higher prevalence of atopy throughout childhood and adolescence.
In conclusion, this study confirms that the co-existence of AHR and wheeze during early childhood identifies a population at increased risk of persistent wheezing who might reasonably be described as having persistent asthma. The onset of asthma during adolescence is closely linked to the presence of atopy, particularly atopy manifest before the age of 10 years. Primary prevention of asthma will require strategies to reduce the prevalence of atopy and/or to reduce the proportion of atopic individuals who acquire asthma. Our findings suggest that there are important environmental determinants of persistent asthma and, when these are identified and modified, secondary prevention will be effective.
Acknowledgments
The authors thank Allen & Hanburys, National Health and Medical Research Council of Australia, and the Community Health and Anti-Tuberculosis Association of Australia for financial support. They gratefully acknowledge the invaluable assistance of study coordinators John Dermand and Nathan Brown, and the research assistants for data collection, and thank the participants and their parents for their help and cooperation which made this longitudinal study possible.