Article Text
Abstract
Background In January 2016, clinical TB guidance in the UK changed to no longer recommend screening contacts of non-pulmonary, non-laryngeal (ETB) index cases. However, no new evidence was cited for this change, and there is evidence that screening these contacts may be worthwhile. The objective of this study was to estimate the cost-effectiveness of screening contacts of adult ETB cases and adult pulmonary or laryngeal TB (PTB) cases in London, UK.
Methods We carried out a cross-sectional analysis of data collected on TB index cases and contacts in the London TB register and an economic evaluation using a static model describing contact tracing outcomes. Incremental cost-effectiveness ratios (ICERs) were calculated using no screening as the baseline comparator. All adult TB cases (≥15 years old) in London from 2012 to 2015, and their contacts, were eligible (2465/5084 PTB and 2559/6090 ETB index cases were included).
Results Assuming each contact with PTB infects one person/month, the ICER of screening contacts of ETB cases was £78 000/quality-adjusted life-years (QALY) (95% CI 39 000 to 140 000), and screening contacts of PTB cases was £30 000/QALY (95% CI 18 000 to 50 000). The ICER of screening contacts of ETB cases was £30 000/QALY if each contact with PTB infects 3.4 people/month. Limitations of this study include the use of self-reported symptomatic periods and lack of knowledge about onward transmission from PTB contacts.
Conclusions Screening contacts of ETB cases in London was almost certainly not cost-effective at any conventional willingness-to-pay threshold in England, supporting recent changes to National Institute for Health and Care Excellence national guidelines.
- tuberculosis
- health economist
- bacterial infection
- respiratory infection
Statistics from Altmetric.com
Key messages
What is the key question?
Was National Institute for Health and Care Excellence correct to change its TB clinical guidelines to no longer recommend screening contacts of non-pulmonary TB cases?
What is the bottom line?
It is almost certainly not cost-effective to screen contacts of non-pulmonary TB cases in London at a willingness-to-pay-threshold of £30 000/quality- adjusted life-years, providing strong evidence that the decision to cease recommending screening contacts of non-pulmonary cases was the correct one.
Why read on?
In addition to helping an answer an important policy question that has been questioned by several recent papers, this article provides the first cost-effectiveness analysis of contact tracing in the UK and the first to incorporate non-pulmonary cases and proposes a novel way to evaluate contact tracing effectiveness.
Introduction
Following 4 years of decline, the incidence of TB in England had fallen to 10.2/100 000 in 20161 but is still higher than most other countries in western and northern Europe.2 Contact tracing, the systematic screening of contacts of cases, is a fundamental part of TB control in high-income countries and is highlighted as a key element of the Public Health England/National Health Service England collaborative TB strategy 2015–2020.3 It is also used around the world for other infectious diseases, including Ebola,4 meningococcal disease5 and sexually transmitted infections.6 The aim of contact tracing for TB is threefold: to reduce morbidity and mortality in contacts with TB by finding them sooner; to reduce transmission from those contacts with active TB; and to find contacts with latent M. tuberculosis infection (LTBI) who are eligible for preventive therapy (PT).7
In January 2016, the UK National Institute for Health & Care Excellence (NICE) TB guidelines changed from recommending screening contacts of all cases to only screening contacts of pulmonary or laryngeal TB (PTB) cases. No new evidence was cited to justify this change.8 Although the guidance on whether contacts of non-pulmonary, non-laryngeal cases (ETB) are screened differs between countries,9 10 most advocate not screening contacts of these cases. Neither the Centers for Disease Control and Prevention nor the WHO advocates screening contacts of these cases, although the WHO guidance is mainly aimed at low-income and middle-income countries.11 12
England has a high proportion of cases with non-pulmonary TB (51% in the most recent year), associated particularly with immigrants from the Indian subcontinent.1 13
While ETB cases are typically not infectious, there is evidence that their contacts are more likely to have TB than the general population. Between 2012 and 2015, the prevalence of active TB among contacts of ETB index cases in London was 0.7%,14 compared with 0.027% in the general population.15 Similar patterns are observed in Birmingham,16 17 and in both cities, the prevalence of disease among contacts of ETB cases was higher than the prevalence of disease among migrants eligible for pre-entry screening18 and more than 10 times higher than the NICE threshold for new entrant screening.17 Additionally, studies have shown only 25% of pairs of cases sharing an address in the UK,19 and 20% of case-contact pairs in London20 had different Mycobacterium tuberculosis isolates, implying the risk of disease in household contacts is high irrespective of whether transmission has occurred. This suggests that the fact that ETB cases are not infectious may not be a valid justification for not screening their contacts.
In light of this evidence, key stakeholders have questioned the change in guidance and a cost-effectiveness analysis has been called for.17 To our knowledge, only one previous study has attempted to evaluate the cost-effectiveness of contact tracing,21 and no studies have done so in the UK or London nor have any studies attempted to evaluate the cost-effectiveness of contact tracing delineated by site of disease of the index case. In this study, we aim to evaluate the effectiveness and cost-effectiveness of contact tracing, for ETB and PTB index cases, in London. We first estimate symptomatic periods and the number of contacts found with active disease or LTBI per index case. We then use these values alongside previously published data to develop a simple static model to calculate the cost-effectiveness.
Methods
Data analysis
We used data on adult and adolescent (>15 years old) TB cases notified to the London TB register (LTBR) during 2012–2015. The LTBR is a web-based register containing demographic and clinical data on all TB cases notified in London since 2002.14 We excluded index cases that were notified in a region and year where the completeness was less than 80% or were children (<14 years old) (because contacts of children with ETB will still be screened under new guidelines).8 When estimating yield, we excluded index cases who first accessed healthcare through contact investigation, as the number of contacts is not recorded consistently.14 Further details of exclusions and the representativeness of data are discussed in Cavany et al 14 (see table 1 in that paper in particular), but demographic characteristics were similar between included and excluded data. Costs were calculated based on national accounting expenditures and current treatment guidance for England8 22 (see online supplementary appendix part 1 for details). Note that, in this manuscript, ETB refers exclusively to non-pulmonary, non-laryngeal TB, and so patients with pulmonary and/or laryngeal TB are classified in PTB, irrespective of whether they have involvement in other organs.
Supplemental material
Variables and constants from other sources
Other data sources
Estimates of utility scores were taken from Jit et al.23 The life-time risk of developing disease following infection was taken from Sloot et al,24 and the efficacy of PT was taken from Smieja et al 25 and Ayieko et al.26 See table 1 for details of data sources.
Effectiveness
We quantified the effectiveness of contact tracing with four outcomes:
Morbidity: the reduction in time contacts with TB are symptomatic if they are found earlier due to contact tracing.
Prevention: the number of contacts with LTBI prevented from developing active TB following PT.
Transmission: the number of cases prevented by reducing transmission from: (A) contacts with prevalent TB found earlier through contact tracing; and (B) cases prevented from occurring due to PT.
Mortality: the number of TB deaths prevented by contact tracing.
Model description
We developed a simple static model to estimate the cost-effectiveness of screening contacts of ETB and PTB cases in London during the period 2012–2015. The model was used to calculate the four measures of effectiveness and estimate the quality-adjusted life-years (QALYs) gained by contact tracing using the following equations (see table 1 and table 2 for definitions of symbols).
Estimates of parameters calculated from the LTBR
In all equations,
σ
is either
P
or
E
and represents the site of disease of the index cases under analysis. The number of PTB index cases is given by  , and the number of ETB index cases is given by
, and the number of ETB index cases is given by  , where
, where  is the fraction of all adult cases that have ETB.
is the fraction of all adult cases that have ETB.
The reduction in morbidity was calculated using the number of contacts with TB per index case ( ), the proportion of contacts with TB that have ETB (
), the proportion of contacts with TB that have ETB ( ) and the difference in symptomatic period of cases found through contact tracing and those found through other routes:
) and the difference in symptomatic period of cases found through contact tracing and those found through other routes:

The number of cases of TB prevented by PT, assuming contacts with LTBI are recently infected is:

where 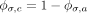 . As the efficacy of PT is different in children (
c
) and adults (
a
), and children are more likely to begin PT than are adults (online supplementary appendix part 2, table G), we calculated the effectiveness of PT separately for these two groups.
. As the efficacy of PT is different in children (
c
) and adults (
a
), and children are more likely to begin PT than are adults (online supplementary appendix part 2, table G), we calculated the effectiveness of PT separately for these two groups.
The number of cases of TB prevented by reducing transmission from contacts with PTB by finding them sooner is:

The prevention of subsequent generations of TB cases that would have occurred in the absence of contact tracing is given by  (see online supplementary appendix part 3).
(see online supplementary appendix part 3).
The number of TB-related deaths prevented by screening contacts is calculated as follows:

where μ is the case fatality ratio. The first term in this equation describes the reduction in mortality among prevalent cases in contacts identified sooner via contact tracing.
To calculate the amount of onward transmission from prevented cases, we assumed a range of values for the number of new infections per PTB case per month infectious, r , and explored the dependence of results on this parameter. This parameter, r , can be related to the updated Styblo rules developed by Trunz et al and van Leth et al 27 28; these studies calculated that each case of smear positive TB would lead to approximately 3–6 new infections, equating to a value of r between 0.5 and 1 (see online supplementary appendix part 4).
Cost-effectiveness
Costs were calculated from a health system perspective. We excluded diagnostic and treatment costs of contacts with TB, as we assumed these contacts would be treated later regardless of whether the contact investigation took place. However, we subtracted the costs of diagnosis and treatment of cases that are prevented. We assumed latently infected contacts are given a 3-month course of rifampicin and isoniazid (with pyridoxine)8 and assumed this has the same efficacy as 6 months of isoniazid.29
We calculated the resulting incremental cost-effectiveness ratio (ICER) for contact tracing of both PTB and ETB index cases, using no screening as the baseline comparator for both. Equations for these calculations are given in the online supplementary appendix part 3. Following NICE recommendations, we assumed a an ICER greater than £20 000–£30 000/QALY was cost-effective30this is the threshold often used in NICE guidance to determine whether an intervention is cost-effective and is also known as the ‘willingness-to-pay’ threshold. We included secondary cases that occurred at any time after infection but assumed most occur in the first year.24 Consequently, most costs and QALY gains occurred in the first year, and so no discounting was included in the main analysis (see online supplementary appendix part 5 for a discussion of discounting).
Uncertainty and sensitivity
As shown in table 1 and table 2, 95% CIs were calculated by randomly selecting 10 000 parameter sets from the distributions. Correlation coefficients were calculated between the distribution of each parameter and distribution of the ICER.
We explored the sensitivity to the symptomatic period by doubling each of these periods and to assumptions about risk of disease following infection and PT by using estimates of these from Erkens et al 31 instead of the estimates from Sloot et al.24
We explored sensitivity to utility scores by using values from Mears et al.23 These were derived from the same source32 as those of Jit et al 33 used in our primary analysis but differ as the Jit et al values were based on London specific data.
Additional analyses
We undertook an additional analysis to estimate the cost-effectiveness of screening of ETB cases that have pleural TB, because it has been reported that 55% of patients with pleural involvement according to X-ray are culture positive on induced sputum.34 We also examined whether there were differences in the cost-effectiveness of screening contacts of UK-born and non-UK-born ETB cases, due to the large differences in the proportion of cases that are ETB between these two groups (51.4% vs 31.9%, respectively.1
Role of finding source
The funding sources played no part in the study design, data analysis, writing of the manuscript or decision to submit for publication.
Results
Mean symptomatic periods
During the period 2012–2015 in London, there were 5084 PTB cases, of whom 2465 met the inclusion criteria and had data on symptomatic period. Of these, 82 were found through contact tracing and were symptomatic for a mean period of 76.6 days (95% CI 58.5 to 94.7). Those who accessed care through other routes were symptomatic for a longer mean period of 110 days (95% CI 103 to 117 days) (p=0.0016) (table 2).
During the same period, there were 6090 ETB cases, of whom 2559 were included and had data on symptomatic period. Of these, 26 were found through contact tracing and had a mean symptomatic period of 152 days (95% CI 15.0 to 289 days). Those who accessed care through other routes had a mean symptomatic period of 180 days (95% CI 165 to 195 days) (p=0.36). See online table E in supplementary appendix part 2 for further details.
Preventive therapy
Of 1497 contacts with LTBI identified in the study period, 1165 (77.8% (95% CI 74.9% to 80.7%) started PT and 918 of those that started (78.6% (95% CI 75.4% to 81.8%) completed PT (table 2). See online table G in supplementary appendix part 2 for further details; of note is that children are much more likely than adults to start PT and, for contacts of PTB cases, to complete PT.
Effectiveness
Reduction in morbidity of contacts
On average, in a single year, not screening contacts of adult ETB cases would have led to those contacts with TB being undiagnosed for a combined additional 2.58 years (95% CI 0.660 to 8.59) (table 3). For contacts of PTB cases, this would be 10.5 years (95% CI 4.02 to 26.4).
Summary of the effectiveness measures included, costs incurred, quality-adjusted life-years (QALYs) gained and resulting incremental cost effectiveness ratio (ICER) for screening contacts of the indicated index cases compared to a baseline of not screening those contacts
Cases prevented by PT
By giving PT to contacts of ETB cases, we would expect to prevent 5.45 (95% CI 3.71 to 7.59) cases. This value would be 18.9 (95% CI 13.1 to 25.8) cases prevented by giving PT to contacts of PTB index cases.
Cases prevented by reduced transmission from contacts
Finding contacts of ETB index cases with TB sooner via contact tracing, thereby reducing onward transmission, could prevent 1.71 cases (95% CI 0.584 to 3.33) when  new infections per PTB case per month infectious. The corresponding value for PTB index cases is 8.76 (95% CI 3.56 to 14.9). This reduction in cases is directly proportional to the assumed value of
r
.
new infections per PTB case per month infectious. The corresponding value for PTB index cases is 8.76 (95% CI 3.56 to 14.9). This reduction in cases is directly proportional to the assumed value of
r
.
Prevention of subsequent generations of cases
Preventing cases from occurring amongst contacts of contacts of ETB cases could avert 1.62 cases (95% CI 0.772 to 3.11) when  and 5.19 cases (95% CI 2.08 to 12.2) when
and 5.19 cases (95% CI 2.08 to 12.2) when  . The corresponding figures for PTB index cases are 8.63 (95% CI 4.77 to 14.7) and 33.1 (95% CI 16.1 to 66.7).
. The corresponding figures for PTB index cases are 8.63 (95% CI 4.77 to 14.7) and 33.1 (95% CI 16.1 to 66.7).
Reduction in mortality
When  , screening contacts of ETB cases could prevent 0.551 deaths (95% CI 0.303 to 1.14) and screening contacts of PTB cases 2.27 deaths (95% CI 1.36 to 3.94).
, screening contacts of ETB cases could prevent 0.551 deaths (95% CI 0.303 to 1.14) and screening contacts of PTB cases 2.27 deaths (95% CI 1.36 to 3.94).
Cost-effectiveness
The cost per QALY of screening the contacts of ETB cases is £101 000/QALY (95% CI 46 200 to 178 000) when transmission is not included ( ), £77 700/QALY (95% CI 38 800 to 139 000) for
), £77 700/QALY (95% CI 38 800 to 139 000) for  new infection per PTB case per month infectious and £56 400/QALY (95% CI 29 300 to 102 000) for
new infection per PTB case per month infectious and £56 400/QALY (95% CI 29 300 to 102 000) for  (table 3, figure 1A). The equivalent values for PTB cases are £43 700/QALY (95% CI 23 700 to 70 100), £30 300/QALY (95% CI 17 700 to 50 100) and £18 700/QALY (95% CI 10 500 to 32 700), respectively (figure 1B). Screening contacts of ETB cases becomes cost-effective at a £30 000/QALY threshold when
(table 3, figure 1A). The equivalent values for PTB cases are £43 700/QALY (95% CI 23 700 to 70 100), £30 300/QALY (95% CI 17 700 to 50 100) and £18 700/QALY (95% CI 10 500 to 32 700), respectively (figure 1B). Screening contacts of ETB cases becomes cost-effective at a £30 000/QALY threshold when  . If
. If  , the yield of ETB index cases would need to be 0.0959 (an almost fivefold increase above the observed yield and greater than current PTB yield) in order for screening contacts of ETB cases to become cost-effective at £30 000/QALY.
, the yield of ETB index cases would need to be 0.0959 (an almost fivefold increase above the observed yield and greater than current PTB yield) in order for screening contacts of ETB cases to become cost-effective at £30 000/QALY.
Summary of incremental cost-effectiveness ratios and 95% CIs (shaded region) for different levels of transmission from contacts. The comparator is no screening. The dashed horizontal line indicates the £30 000/QALY cost-effectiveness threshold, and the dotted horizontal line indicates the £20 000/QALY threshold. The solid horizontal line indicates when contact tracing becomes cost-saving. Figure parts A and B represent the main results for ETB and PTB index cases, respectively. Figure parts C and D represent results for a symptomatic period, which is double the self-reported period. ETB, non-pulmonary, non-laryngeal TB; GBP, pounds sterling; ICER, incremental cost-effectiveness ratio; PTB, pulmonary or laryngeal TB; QALY, quality-adjusted life-years.
Sensitivity
Cost-effectiveness results are most sensitive to the symptomatic period of those found through contact tracing (online supplementary appendix table H) (especially of contacts of ETB index cases), the probability of developing disease and the yield of ETB index cases. At low levels of transmission from PTB contacts, the symptomatic period of contacts with ETB explains most of the variation in the ICER. As the number of infections generated by contacts is increased, the results become more sensitive to the probability of developing disease and the symptomatic period of PTB index cases and less sensitive to the symptomatic period of ETB index cases. Increasing each symptomatic period by a factor of 2 (figure 1C,D), then for  , the mean cost-effectiveness of screening contacts of ETB cases is below the £30 000/QALY threshold. Calculating the probability of developing disease from Erkens et al
7 rather than Sloot et al
24 does not qualitatively change the cost-effectiveness results (not shown). Using utility scores used by Mears et al
23 instead of those used by Jit et al
33 leads to a slight decrease in cost-effectiveness (online supplementary appendix part 6).
, the mean cost-effectiveness of screening contacts of ETB cases is below the £30 000/QALY threshold. Calculating the probability of developing disease from Erkens et al
7 rather than Sloot et al
24 does not qualitatively change the cost-effectiveness results (not shown). Using utility scores used by Mears et al
23 instead of those used by Jit et al
33 leads to a slight decrease in cost-effectiveness (online supplementary appendix part 6).
Additional analyses
While screening contacts of pleural TB cases is more cost-effective than screening contacts of other ETB cases, it still appears to be probably not cost-effective at a threshold of £30 000/QALY for values of r less than 3 (online supplementary appendix figure B).
Similarly, If we restrict our analysis to UK-born cases only, then screening contacts of ETB cases is probably not cost-effective at a threshold of £30 000/QALY for values of r below 3 (online supplementary appendix figure C). It is also unlikely to be cost-effective to screen contacts of non-UK-born ETB cases for values of r below 4. For PTB cases, it is probably cost-effective to screen contacts of non-UK-born PTB cases at a threshold of £30 000/QALY when r is greater than 1.65 (online supplementary appendix figure C). Screening contacts of UK born PTB cases is probably cost-effective at £30 000/QALY even if no transmission takes place and becomes probably cost-effective at £20 000/QALY when r is greater than 0.834.
Discussion
Principal findings
On average, we estimate that in a single year, screening contacts of ETB would save a total of 2.58 years of morbidity in contacts with prevalent TB and prevent at least 5.45 cases through reduced transmission and PT. However, screening ETB contacts was very unlikely to be cost-effective at a threshold of £30 000/QALY, even with the assumption of high levels of transmission from contacts. Hence, the results presented here support recent changes to the NICE guidelines to remove screening of contacts of ETB cases from their guidance. In contrast, screening contacts of PTB cases was probably cost effective at a £30 000/QALY threshold, especially when assuming high levels of transmission from contacts. Neither was likely to be cost-effective at a £20 000/QALY threshold at plausible levels of transmission.
Strengths and limitations
This study used high-quality data on contact tracing yield in London to answer an important question for TB care and prevention, which has implications for TB policy in the UK. The approach used proposes a novel way of quantifying the effectiveness of contact tracing across four potential impacts (reduced morbidity, PT, reduced transmission and reduced mortality). The main limitation of the study is the large uncertainty in several parameters. However, we explored this first by varying the number of infections generated by each case ( r ) and by carrying out a probabilistic sensitivity analysis of all other parameters. A related limitation is the treatment of transmission. It is difficult to know the rate at which infectious contacts would infect further contacts, so we explored a range of assumptions. We did not characterise the indirect effect of contact tracing on transmission at a population level, though as only 5% of all cases in London are found through contact tracing, this is probably negligible over short timescales. The quantitative nature of this approach is unable to assess broader outcomes of contact tracing, such as community engagement and tackling stigma. Finally, we used the self-reported symptomatic period to estimate the time during which cases are infectious. Due to issues with patient recall and the fact that the ratio of estimated prevalence to incidence in London15 35 is much greater than the mean self-reported symptomatic period found in this study, it is likely that this value systematically underestimates the true time people are symptomatic. Our sensitivity analysis showed that cost-effectiveness of contact tracing would increase, and screening contacts of ETB cases would be possibly cost-effective at a £30 000/QALY threshold if the symptomatic period was double that estimated by self-reported symptom onset (figure 1C,D).
Our approach should not suffer from selection bias as, although we only included those cases and contacts detected by healthcare; in this case, we are interested in the actual effect that would be experienced by the healthcare system, and so we are only interested in those cases and contacts that are actually found. While we did exclude some regions and time-periods from the underlying dataset due to large amounts of missing data (see Cavany et al for details14), meaning some ascertainment bias may have been present, the excluded cases had similar demographic characteristics to those included. It is also possible some differential bias may have been present if cases were incorrectly classified as ETB or PTB, which is possible as a 24% of PTB cases and 51.9% of extrapulmonary cases were not culture confirmed in 2017 in England.1
Relation to other studies
In recent years, studies in the UK have evaluated the cost-effectiveness of screening new migrants36 and hard-to-reach populations using a mobile X-ray unit (MXU, known as Find & Treat).33 In 2011, Pareek et al
36 found that screening migrants from countries with an incidence exceeding 150/100 000 cost £21 000 per case averted. This is cheaper than screening ETB contacts and similar to screening PTB contacts for  new infections per PTB case per month infectious (table 3). Jit et al found that screening hard-to-reach groups in London cost £6400–£10 000/QALY gained, so it was more cost-effective than screening PTB cases even if
new infections per PTB case per month infectious (table 3). Jit et al found that screening hard-to-reach groups in London cost £6400–£10 000/QALY gained, so it was more cost-effective than screening PTB cases even if  . In their study, Jit et al
33 found that about 80% of QALYs gained were due to improved case management of these complex cases, and the cost-effectiveness of screening alone was similar to screening contacts of PTB cases. The case management impact would likely be smaller for contact tracing than for the MXU, because the population of contacts is less complex, and case management is not an explicit aim of contact tracing. When Dasgupta et al
21 compared the cost-effectiveness of screening close contacts to migrant screening in Montreal, they found that close contact investigation was cost saving. This was due to much lower treatment costs of contacts as opposed to cases found through other routes, due largely to much higher rates of hospitalisation among passively detected cases. However, this assumption was based on only six cases found through contact tracing. We did not explore the impact of decreased hospitalisation rates here due to a lack of data. Finally, a 2008 study in British Columbia, Canada,37 found that giving PT to contacts was cost-effective, though this study focused on infectious index cases. Our results are not directly comparable with this study due to its focus on PT but both support the continued screening of contacts of PTB cases.
. In their study, Jit et al
33 found that about 80% of QALYs gained were due to improved case management of these complex cases, and the cost-effectiveness of screening alone was similar to screening contacts of PTB cases. The case management impact would likely be smaller for contact tracing than for the MXU, because the population of contacts is less complex, and case management is not an explicit aim of contact tracing. When Dasgupta et al
21 compared the cost-effectiveness of screening close contacts to migrant screening in Montreal, they found that close contact investigation was cost saving. This was due to much lower treatment costs of contacts as opposed to cases found through other routes, due largely to much higher rates of hospitalisation among passively detected cases. However, this assumption was based on only six cases found through contact tracing. We did not explore the impact of decreased hospitalisation rates here due to a lack of data. Finally, a 2008 study in British Columbia, Canada,37 found that giving PT to contacts was cost-effective, though this study focused on infectious index cases. Our results are not directly comparable with this study due to its focus on PT but both support the continued screening of contacts of PTB cases.
Interpretation of results
These results support the recent decision to remove screening contacts of adult ETB cases from NICE guidance. In order for screening these contacts to be cost-effective at a £30 000/QALY threshold, r would need to be 3.40 new infections per PTB case per month infectious, which would mean each smear positive case would need to generate 21 new infections. This is likely to be high for some settings27 but may be plausible in crowded environments, such as homeless shelters.38 Additionally, we found that if the yield per ETB index case was above 0.0959, then the ICER for screening contacts of these cases was below £30 000/QALY. In London, ETB cases with a history of homelessness or drug use have a yield greater than this (unpublished data), supporting recommendations for active case-finding among this group. Additionally, subgroups for whom the yield is higher are also those for whom r is likely to be higher, further increasing the impact of screening contacts of those subgroups. It is unlikely that the average yield of ETB cases in other parts of the UK are much higher than those seen in London,16 implying that it would also not be cost-effective to screen contacts of ETB cases nationally.
If we stratify our data into UK-born and non-UK-born groups, we see that it is more cost-effective to screen contacts of UK born PTB cases than it is non-UK-born PTB cases (online supplementary appendix figure C). This is in part due to the much greater difference in symptomatic period between those found through contact tracing and those found through other routes for UK-born cases compared with non-UK-born cases (online supplementary appendix table M and N). This implies the gap in cost-effectiveness of contact tracing for UK-born cases compared with non-UK-born cases could be closed if contact tracing found non-UK-born cases more quickly. The caveat to this result is that there is an assumption that contacts of UK born cases are also UK born, and non-UK-born cases are non-UK born, which is not true, and which means we underestimate the impact of contact screening for non-UK-born cases.
The impact on the ICER caused by changing the amount of transmission ( r ) indicates the importance of reducing transmission from contacts as one of the impacts of contact tracing. It is plausible, though, that the number of infections generated by a contact with PTB (ie, the value of r ) will be lower than that suggested by the re-estimated Styblo rule,27 28 as the household contacts of someone themselves found through contact tracing are more likely to have already been infected.
The main reason for the low ICER for ETB index cases was the small difference in symptomatic period of contacts with ETB and cases with ETB found through other routes (online supplementary appendix table H), suggesting that the impact may be improved by hastening contact tracing for these contacts. The NICE guidelines now recommend PT for anyone aged under 65 years. This may cause a small improvement in cost-effectiveness, as we would now expect a higher yield of LTBI per case, as more contacts will be tested for LTBI, provided it is not accompanied by lower rates of PT enrolment and completion. The introduction in 2017 of whole genome sequencing (WGS) in the UK39 may also affect our conclusions. While a study of the current strain typing service found no impact on contact tracing,23 it is plausible that faster turnaround times and improved targeting available with WGS may affect contact tracing yields.
Further research
This work would benefit from an improved understanding of the rate of onward transmission from contacts. Mathematical modelling work incorporating transmission on a network structure may help to understand this. It would also help to have a greater understanding of the proportion of contacts that have pulmonary TB and how this differs across groups. If there are subgroups for whom a greater than average proportion of contacts with TB have PTB, then this would increase the cost-effectiveness in these groups. While we were able to estimate this proportion for the whole population, our small sample meant we could not stratify this estimate. Work to understand how the different screening approaches (migrant, hard-to-reach populations and contacts) interact would help our understanding of the impact of each. Our results were very sensitive to estimates of the symptomatic period of contacts, both due to the uncertainty of these estimates and the fact that they are based on self-reported periods. A more thorough understanding of diagnostic delay among both contacts and non-contacts is needed.
Acknowledgments
We would like to thank Jacqui Carless, Lamya Kanfoudi and others at PHE field Epidemiology London for maintaining the LTBR; all nurses and other healthcare workers involved with collecting the data and presenting it at cohort review; the TB modelling group at LSHTM for advice on presentation and approach; and Lindsay Serene for comments on the manuscript.
References
Footnotes
Contributors SMC, EV and TS conceived and designed the work, with input from all other authors. CSA and HM are responsible for the acquisition and maintenance of the data. SMC undertook the analysis with advice from all other authors. FS calculated the costs. All authors contributed to the interpretation of the data. SMC wrote the first draft of the paper and all authors contributed to subsequent drafts. All authors approve the work for publication and agree to be accountable for the work.
Funding SMC and FS are funded separately by a joint PHE-LSHTM studentship in Infectious Disease Modelling. RGW is funded by the UK Medical Research Council (MRC) and the UK Department for International Development (DFID) (under the MRC/DFID Concordat agreement that is also part of the EDCTP2 programme supported by the European Union (MR/J005088/1)), the Bill and Melinda Gates Foundation (TB Modelling and Analysis Consortium: OPP1084276 and SA Modelling for Policy: #OPP1110334) and UNITAID (4214-LSHTM-Sept15; PO #8477-0-600). CSA, EV, HM and HLT are employed by PHE, a government agency, and received no other source of funding. TS is funded by the Bill and Melinda Gates Foundation (SA Modelling for Policy: #OPP1110334). SMC made the final decision to submit and had access to all data.
Competing interests None declared.
Patient consent Not required.
Ethics approval Ethical approval was not required. The data analysed were routinely collected surveillance data held by PHE under Section 251 of the NHS Act 2006. All records were anonymised before analysis.
Provenance and peer review Not commissioned; externally peer reviewed.



