Article Text
Abstract
Background Matrix metalloproteinases (MMPs) are proteolytic enzymes that can degrade the extracellular matrix and drive tissue remodelling, key processes in the pathogenesis of COPD. The development of small airway disease has been identified as a critical mechanism in the early development of airflow obstruction but the contribution of MMPs in human disease is poorly characterised.
Objectives We investigated the role of MMPs and inflammatory cytokines in the lung by quantifying levels and determining relationships with the key pathological components of COPD in patients and healthy controls.
Methods We analysed levels of MMPs and inflammatory cytokines in bronchoalveolar lavage from 24 COPD and 8 control subjects. Each subject underwent spirometry and high-resolution CT. Image analysis quantitatively assessed emphysema, bronchial wall thickening and small airways disease.
Results Multiple MMPs (MMP-1, -2, -3, -8, -9 and -10) and cytokines (interleukin (IL) 6 and IL-8) were elevated in lungs of subjects with COPD. MMP-3, -7, -8, -9, -10 and -12 concentrations closely associated with CT markers of small airways disease. Emphysema severity was also associated with MMP-3, -7 and -10. However, there were no strong relationships between MMPs and bronchial wall thickness of the larger airways.
Conclusions Pulmonary MMP concentrations are directly associated with the extent of gas trapping and small airways disease identified on CT scan. This study suggests that MMPs play a significant role in small airways remodelling, a key feature in the pathogenesis of COPD.
Trial registration number NCT01701869
- COPD ÀÜ Mechanisms
- Lung Proteases
- Imaging/CT MRI etc
- Emphysema
Statistics from Altmetric.com
Key messages
What is the key question?
Are matrix metalloproteinases (MMPs) involved in the development of pathological changes in the lung of patients with COPD and what is their association with emphysema formation, bronchial wall thickening and small airways remodelling?
What is the bottom line?
Bronchoalveolar lavage concentrations of multiple MMPs are increased in patients with COPD, and these are associated with the degree of small airways disease and emphysema measured by CT analysis.
Why read on?
This study combines quantitative CT analysis with multiplex profiling of MMPs and inflammatory mediators to identify a new role of proteases in COPD.
Introduction
COPD is a heterogeneous disease characterised by the progressive development of airflow limitation which leads to functional impairment and associated symptoms.1 The underlying mechanisms of disease are poorly understood, which has limited the development of new therapeutic and diagnostic approaches.2
Tissue destruction, inflammation and airway remodelling are important features in COPD, and a number of pathways and mediators have been implicated in these processes.3 A proteinase/antiproteinase imbalance has been postulated to be a key contributor to emphysematous changes.4 Matrix metalloproteinases (MMPs), a large family of zinc-dependent proteolytic enzymes, have the ability to degrade the pulmonary extracellular matrix (ECM) and have been implicated in COPD.4 They are broadly grouped depending on their substrate specificity, including collagenases (MMP-1, -8 and -13), gelatinases (MMP-2 and -9), stromelysins (MMP-3, -10 and -11) and elastases (MMP-7 and -12),5 although there is considerable substrate overlap. MMPs are not normally expressed in healthy tissue but in disease can be produced by alveolar macrophages, neutrophils and bronchial epithelial cells.5 They are tightly regulated by specific endogenous inhibitors, the four Tissue inhibitors of MMPs (TIMP).5
Transgenic mice over expressing MMP-1 develop emphysema at an accelerated rate.6 Selective inhibition of MMP-9 and -12 in guinea pigs reduced the extent of emphysema following exposure to smoke.7 Human studies demonstrate increased expression of MMP-1,8 ,9 -8,9 ,10 -98–13 and -128 ,14 ,15 in the sputum or bronchoalveolar lavage (BAL) of subjects with COPD. However, detailed analysis of the MMP profile has not been integrated with systematic characterisation of lung pathology by high-resolution CT and lung function.
MMPs have complex biology with multistep activation and numerous molecular interactions,16 and the most important MMPs have yet to be determined in COPD. In addition, MMPs have multiple other functions that are independent of their ability to degrade the ECM, including facilitating cell migration and activating growth factors and cytokines.5 Most studies investigating MMPs in COPD have only investigated a subset of MMPs. Given their biological complexity, novel multiplex-based arrays provide the opportunity to profile a broad spectrum of MMPs in a carefully characterised clinical cohort.
The key sites of MMP activity in the lung are unknown and it is likely that matrix turnover and remodelling differs between regions. CT imaging provides the opportunity to study key morphological features of COPD including emphysema, bronchial wall morphology and small airways disease. Quantitative analysis software allows numerical, objective estimation of these disease facets.17 MMP-9 and -12 have been shown to have associations with quantitative measures of emphysematous change on CT.11 ,14 Previous studies have not investigated the association between MMP concentrations and bronchial wall measures or small airways disease identified by CT. In this study, we combine CT analysis of lung pathology with multiplex profiling of MMPs and inflammatory mediators, and identify a novel role for MMPs in COPD.
Methods
Subjects
Subjects gave written informed consent and the study (ClinicalTrials.gov:NCT01701869) was approved by the South Central—Southampton B NRES Committee (12/SC/0304).
Twenty-four subjects with stable mild and moderate COPD as defined by GOLD guidelines1 were recruited into the study. Postbronchodilator spirometry was used to assess airflow obstruction with an FEV1:FVC ratio of <0.7 and an FEV1 of ≥50% predicted value required for enrolment. Spirometry was conducted in accordance with American Thoracic Society standards.18 Subjects had at least a smoking history of 10 pack years. Exclusion criteria included a history of other pulmonary disease, α-1-antitrypsin deficiency, long-term antibiotics/steroids or an exacerbation within the month prior to recruitment. A control group of eight current or ex-smokers, with at least a 10 pack year history but preserved lung function were also recruited.
CT scanning and quantitative image analysis
Subjects underwent volumetric CT scans of the chest using a Siemens Sensation 64 CT scanner. The imaging protocol consisted of; slice thickness 0.75 mm, slice separation 0.5 mm, tube voltage 120 KV, effective mAs 90 mAs (using dose modulation), collimation 0.6 mm and a pitch of 1. Subjects were scanned at full inspiration. A subcohort of 22 subjects was scanned at end-tidal volume. The remaining did not have an expiratory scan due to concerns over cumulative radiation exposure having had recent CT scans.
Images reconstructed with the B35 kernel were used for image analysis using Apollo Software (VIDA Diagnostics). Emphysema was quantified by the per cent of lung voxels on the inspiratory scan with attenuation values below −950 HU (%LAA). Bronchial wall thickening was quantified using the standardised parameter Pi10, which is the square root of the wall area of a hypothetical airway with a 10 mm internal perimeter. A surrogate marker for small airways disease was measured using the ratio of mean lung attenuation on expiratory and inspiratory scans (E/I MLD), which has been previously validated.19
Bronchoscopy and sample acquisition
Fibre optic bronchoscopy was performed on an outpatient basis. In each subject, two lobes were targeted, and BAL was performed by instilling 100 mL of 0.9% saline into each lobe and recovered by aspiration. BAL fluid was poured through 100 µm filters and cells removed by 400 g centrifugation for 10 min at 4°C. The supernatant was aliquoted and stored at −80°C prior to analysis. The resulting cell pellets were prepared for cytospin analysis as previously described.20
MMP and cytokine analysis
MMP and cytokine concentrations in BAL were quantitated using a microparticle-based multiplex immunoassay (R&D systems, Abingdon, UK) developed by Luminex. Samples were analysed on the Luminex 200 platform (Biorad Bioplex 200, Hemel Hempstead, UK), as per manufacturer's instructions. The following MMPs were analysed: MMP-1, -2, -3, -7, -8, -9, -10, -12, -13 and ECM metalloproteinase inducer (EMMPRIN), a cell surface molecule that can be shed. TIMP 1–4 were analysed and cytokine analysis was performed for interleukin (IL) 1β, IL-2, IL-6, IL-8, IL-10, GM-CSF, IFNγ and TNFα (see online supplementary data for assay sensitivities).
Statistical analysis
Statistical analyses were performed using SPSS V.21. Mann–Whitney U test and Fisher's exact tests compared data between COPD and control groups. In the subjects with COPD, associations between MMPs, spirometry, CT parameters and cytokines were assessed using Spearman's correlation with r and p values presented. Partial Spearman's correlation was used to conduct multivariate analysis. Each subject had two lobes sampled, and the mean concentrations between the lobes were used. For the purpose of statistical analysis, values that were below the lower limit of detection were given the value of half the concentration of detection. A p value of <0.05 was considered statistically significant.
Results
Subject characteristics
COPD and control groups were well matched for age, sex and current smoking status (table 1). There were more males in both groups. FEV1% and FEF25%–75% were significantly lower in the COPD group.
Characteristics of participants included in the study
MMP concentrations in COPD and controls
Median MMP-1, -2, -3, -8, -9 and -10 were significantly higher in the BAL of subjects with COPD compared with controls (figure 1). There was no significant difference in MMP-7, -12, -13 and EMMPRIN between the groups.
Bronchoalveolar lavage (BAL) expression of matrix metalloproteinases (MMPs) in COPD and controls (HC). (A) MMP-1, (B) MMP-2, (C) MMP-3, (D) MMP-7, (E) MMP-8, (F) MMP-9, (G) MMP-10, (H) MMP-12, (I) MMP-13, (J) extracellular matrix metalloproteinase inducer (EMMPRIN). Data represents median with IQR. Each dot represents BAL concentration of individual MMP in a specific patient, n=24 for COPD and 8 for controls. *p<0.05, **p<0.01, ***p<0.001 using Mann–Whitney U test.
Neutrophil and cytokine concentrations in COPD and controls
BAL neutrophil count, IL-6 and IL-8 concentrations were significantly higher in subjects with COPD than controls (figure 2). There were no significant differences in IL-1β and GM-CSF between groups. IL-2, IL-10, IFNγ and TNF-α were only detected at very low concentration in all samples and so further analysis was not performed.
Bronchoalveolar lavage (BAL) neutrophils and cytokines in COPD and controls (HC). (A) differential BAL neutrophil count (%), (B) IL-1β, (C) IL-6, (D) IL-8, (E) GM-CSF. Data represent median with IQR. Each dot represents BAL concentration of individual value in a specific patient, n=24 for COPD and 8 for controls. *p<0.05, **p<0.01 using Mann–Whitney U test. IL, interleukin.
Associations between MMPs, cytokines, lung function and CT parameters
We investigated the relationships between MMPs, cytokines and physiological and CT parameters in the subjects with COPD.
Relationships of MMPs with cytokines and neutrophils
We performed a systematic analysis of associations between cytokines, neutrophils and tissue-destructive MMPs, using Spearman's correlation. There were significant associations between the cytokines IL-6 and IL-8 and MMP-1, -7, -8, -9, -12 and EMMPRIN (table 2). GM-CSF was associated with MMP-8, -9 and EMMPRIN. IL-1β had a negative correlation with MMP-3 while neutrophil counts were not associated with any MMPs.
Spearman's correlation analysis between matrix metalloproteinases (MMPs), spirometry, CT measures of disease and cytokines and neutrophils in subjects with COPD
Relationships of MMPs, cytokines, neutrophils with lung function
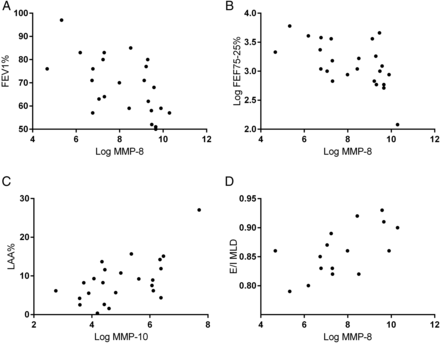
We compared MMP and cytokine concentrations with lung function markers of disease severity using Spearman's correlation. There were significant associations between airflow obstruction (FEV1%) and MMP-8, -9 and -12 and between FEF25%–75% and MMP-1, -8, -9 and -12 (table 2). MMP-8 had the strongest association with both FEV1% and FEF25%–75% (figure 3) and using partial correlation this remained significant when adjusting for each of the other MMPs in turn apart from MMP-9 (see online supplementary table S1).
Scatter plots of subjects with COPD for (A) log matrix metalloproteinase (MMP)-8 against FEV1%, (B) log MMP-8 against log FEF25%−75%, (C) log MMP-10 against LAA%, (D) log MMP-8 against E/I MLD. These plots are visualised as they represent the MMPs with the strongest associations with the accompanying outcome variable. N=24, apart from E/I MLD (n=16).
FEV1% and FEF25%–75% were significantly associated with IL-8. FEF25%–75% was also associated with IL-1β. There was no association between these spirometric markers and BAL neutrophils, IL-6 and GM-CSF (see online supplementary table S2).
CT analysis
Quantitative CT analysis was performed to study emphysema, bronchial wall morphology and small airways disease and assess the relationships with MMPs and cytokines. Segmentation and quantitative analysis was successfully achieved in 31 subjects. One of the control CT scans could not be analysed for technical reasons.
Emphysema
Subjects with COPD had significantly more emphysema (higher %LAA) than controls (table 1), although there was no significant association between emphysema and FEV1% in the subjects with COPD (r −0.10, p=0.63).
There were significant associations between emphysema (%LAA) and MMP-3, -7 and -10 (table 2). MMP-10 had the strongest association with emphysema (figure 3) and using partial correlation this remained significant when adjusting for each of the other MMPs apart from MMP-3 and -7 (see online supplementary table S1). Emphysema was not associated with neutrophils or cytokines (see online supplementary table S2).
Small airways disease
Small airways disease (E/I MLD) was greater in the COPD group than controls (table 1). Strong associations with FEV1% (r −0.72, p=0.002) and FEF25%–75% (r −0.54, p=0.03) were seen in subjects with COPD.
There were associations between small airways disease (E/I MLD) and MMP-3, -7, -8, -9, -10 and -12 (table 2). MMP-8 had the strongest association with small airways disease (figure 3) and this remained significant after adjusting for MMP-1, -2 and -13 in turn but not the other MMPs (see online supplementary table S1). The only cytokine that correlated with small airways disease was IL-8 (see online supplementary table S2).
Bronchial wall thickening
There was no significant difference in bronchial wall thickening (Pi10) between COPD and controls, but there was an association between Pi10 and FEV1% in subjects with COPD (r −0.59, p=0.003). There were no associations between airway wall area and MMPs (table 2). Neutrophils and cytokines did not show any association with bronchial wall area (see online supplementary table S2).
Discussion
This study has, for the first time, demonstrated an association between small airways disease measured by CT imaging and the lung concentrations of MMPs and inflammatory cytokines in COPD. MMPs and cytokines are also associated with other markers of disease severity, including FEV1% and emphysema severity. The analysis suggests that associations were stronger between MMPs and small airways disease rather than emphysema.
MMPs are structurally similar proteolytic enzymes that have been implicated in the tissue remodelling, matrix destruction and inflammation seen in COPD.4 We found that MMP-1, -2, -3, -8, -9 and -10 were elevated in the airways of subjects with COPD. This is in keeping with previous studies which demonstrated that MMP-1,8 ,9 -89 ,10 and -98–13 are increased in subjects with COPD. Furthermore, we have also shown that MMP-2, -3 and -10 were increased. A histological study of lung resection subjects also found that MMP-2 was upregulated in the lung periphery in COPD.21 However, Culpitt et al9 found no difference between MMP-2 and -3 in sputum between COPD and controls. Our results may diverge from these due to the difference in sampling locations between sputum and BAL. Previous studies also found that MMP-12 is increased in COPD,8 ,14 ,15 although we did not replicate this observation in our cohort. We analysed the ratio of MMPs: TIMPs and found they were significantly increased in subjects with COPD (data not shown) and this was especially the case for MMP-8 and -10. This supports the idea of a proteinase to antiproteinase imbalance; however, further work is required to understand this fully. We also found the concentrations of neutrophils, IL-6 and IL-8 were increased in COPD, which is consistent with previous work.22 This provides further evidence for the role of neutrophilic airway inflammation in COPD.
Most work to date has focused on assessing the role of MMPs in emphysema. Transgenic mice overexpressing MMP-1 develop emphysema at an accelerated rate.6 MMP-12 knockout mice are protected from cigarette induced lung damage.23 In the SMAD3 mouse model of emphysema, genetic blockade of MMP-9 reduced the amount of emphysema.24 Selective inhibition of MMP-9 and -12 in guinea pigs reduced the extent of emphysema when exposed to smoke.7 In human genetic studies, polymorphisms of MMP-9 genes have been linked to emphysematous change25 ,26 while polymorphisms of MMP-12 have been linked to airflow obstruction.27 Previous work using lavage fluid found that MMP-1, -9 and -12 were significantly raised in patients with emphysema when compared with healthy non-smokers but not current smokers.8 In two studies using quantitative CT analysis, the LAA% correlated with sputum MMP-9 and -12.11 ,14 Unlike these two studies, we did not find any associations between CT-measured emphysema and MMP-9 or -12, which may be due to the differences between sampling sputum or BAL. However, we found significant associations between quantified emphysema and MMP-3, -7 and -10, which have not been previously investigated. MMP-10 had the strongest association with emphysema, and interestingly, this and the other significantly associated MMPs have limited elastin degradation properties and mainly degrade collagens and proteoglycans. Studies show that there is actually increased collagen around emphysematous lesions,28 and therefore a key mechanism may be MMP-induced collagen breakdown and subsequent abnormal remodelling and aberrant deposition of collagen.
We measured bronchial wall thickening of larger airways using the standardised marker Pi10 which has been shown to correlate with FEV1%29 and is elevated in COPD.30 Our analysis found no significant difference in Pi10 between COPD and controls but did show an association with airflow obstruction. There were no significant associations between Pi10 and MMPs or inflammatory cytokines. The reasons for this are unknown, but it may be that Pi10 is not the best measure at assessing airway wall morphology. It is also feasible that bronchial wall thickening is not directly linked to luminal inflammatory indices or protease activity and the remodelling response in the proximal airway may be more related to inflammatory infiltration of the submucosa31 and biopsy studies may be required to elicit these mechanisms.
Small airways disease is a key feature of COPD and has been identified as the main site of the airflow obstruction seen in the disease.32 Histopathological studies have identified that the small airways are thickened and morphologically abnormal with a combination of squamous cell metaplasia, goblet cell hyperplasia and peribronchial fibrosis apparent.33 There is currently no gold standard for investigating and assessing small airways disease in COPD. CT lacks the resolution to image the small airways directly; however, the indirect sign of gas trapping can be used.17 A number of quantitative CT methods exist to measure this, and we chose to use the ratio of mean lung density in expiration against inspiration. Hersh et al34 confirmed that E/I MLD had a good correlation with lung function markers of small airways disease. Bommart also demonstrated that E/I MLD was the best CT marker of gas trapping as measured by nitrogen washout.19 We found E/I MLD was significantly raised in COPD and had strong associations with FEV1% and FEF25%–75%, supporting its use as a marker of small airway disease.
Few studies have investigated the role of MMPs in small airways disease. In guinea pigs, inhibition of MMP-9 and -12 protected the animals from small airways fibrosis in response to cigarette smoke exposure.7 A study of symptomatic smokers with normal lung function found that MMP-8, -9 and -12 in induced sputum correlated with FEF25%–75%.35 Our results found that MMP-1, -8, -9 and -12 had associations with FEF25%–75%. However this is an insensitive and non-specific marker of small airways disease. When using the CT marker E/I MLD we found strong associations with the concentrations of MMP-3, -7, -8, -9, -10 and -12. These associations tended to be stronger than those seen with emphysema, suggesting that MMPs may play a significant role in the development of small airway remodelling and the associated airflow obstruction. MMP-8 had the strongest association with small airways disease and along with the other associated MMPs is mainly produced by neutrophils and macrophages and between them are able to degrade all components of the ECM. In the small airways, it has been demonstrated that elastin and collagen are reduced in patients with COPD,36 and we propose that this degradation is driven by MMPs leading to deranged remodelling and fibrosis.
We recognise that the main limitation of this study was the small sample size and associated limited statistical power, meaning that we were unable to analyse certain aetiological factors such as smoking. However, every effort was made to phenotype our cohort with CT imaging and invasive bronchoscopy techniques. Despite this, we found strong evidence of the associations between MMPs and clinical features of disease. Another limitation is the multiple comparisons made in this study. We tested 129 associations between parameters of physiology, CT indices and laboratory markers. At the 5% level, around seven associations would be expected to be significant just by chance. We found 38 significant associations, far higher than the number expected by chance, suggesting the presence of genuine associations. Due to the need to perform bronchoscopy, our study consisted of patients with mild and moderate COPD, with only limited amounts of emphysema. It is unknown whether these results would be the same in more severe patients. In this study, we measured concentrations of MMPs rather than MMP activity. Lowrey demonstrated that although MMP-9 concentrations were increased in the sputum of subjects with COPD, MMP-9 activity was not.12 However, due to the site and nature of action of MMPs, there is considerable debate as to whether concentrations or activity assays best reflect the action of MMPs.37 Another limitation was that only a subcohort of 16 subjects with COPD had both an inspiratory and expiratory CT scan, allowing assessment of small airways disease. Repeating emphysema and airflow analysis on this subsample yielded broadly similar results, indicating the smaller sample size did not influence the results.
In conclusion, multiple MMPs are increased in the airways of subjects with COPD and are associated with the severity of airflow obstruction and quantitative CT measures of emphysema and small airways disease. This suggests that MMPs may play a significant role in the pathogenesis of COPD by causing breakdown of the pulmonary ECM leading to abnormal remodelling in both the small airways and lung parenchyma. Further work is required to investigate these important mechanisms and to understand the heterogeneity of the disease within different compartments of the lung. While most previous work has focused on MMPs and emphysema, this study suggests the strongest associations were with small airways disease. Interventions directed at inhibiting MMPs may have a role in preventing small airways remodelling, and any trial investigating modulation of MMPs should use CT analysis as a marker of small airways disease.
Acknowledgments
We would like to express our appreciation to the staff of the Southampton NIHR Respiratory Biomedical Research Unit, in particular Kate Lippiett, Shuna Egerton, Sukh Purewal and Sarah Bawden. We also acknowledge the important contributions of the GSK vaccine team who provided funding and project management. We extend our gratitude to all the volunteers who gave their time and enthusiasm to make this research possible.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1 - Online supplement
Footnotes
Twitter Follow Simon Bourne at @COPDeducation
Contributors Conception and design: KO, LW, PTE, KJS and TMAW; data acquisition, analysis and interpretation: all authors; drafting of manuscript for important intellectual content: KO, KJS and TMAW.
Funding This study was funded by GSK Biologicals, Belgium via a Collaborative Research and Development Agreement (CRADA). No restrictions were placed on authors regarding the statements made in the manuscript.
Competing interests None declared.
Ethics approval NRES Committee South Central—Southampton B (12/SC/0304).
Provenance and peer review Not commissioned; externally peer reviewed.