Article Text
Abstract
Background: All-trans retinoic acid (ATRA) stimulates elastin synthesis by lung fibroblasts and induces alveolar regeneration in animal models of pulmonary emphysema. However, ATRA treatment has had disappointing results in human emphysema. It was hypothesised that a defect in the ATRA signalling pathway contributes to the defect of alveolar repair in the human emphysematous lung.
Methods: Fibroblasts were cultured from the lung of 10 control subjects and eight patients with emphysema. Elastin and retinoic acid receptor (RAR)-β mRNAs were measured in those cells in the presence of incremental concentrations of ATRA. RARs, retinoic X receptors (RXRs) and cellular retinoic acid binding protein (CRABP) 1 and 2 mRNAs were measured as well as CRABP2 protein content. The effect of CRABP2 silencing on elastin and RAR-β expression in response to ATRA was measured in MRC5 lung fibroblasts.
Results: ATRA at 10−9 M and 10−8 M increased median elastin mRNA expression by 182% and 126% in control but not in emphysema fibroblasts. RAR-β mRNA expression was induced by ATRA in control as well as emphysema fibroblasts. RARs, RXRs and CRABP1 mRNAs were similarly expressed in control and emphysema fibroblasts while CRABP2 mRNA and protein were lower in emphysema fibroblasts. CRABP2 silencing abrogated the induction of elastin but not RAR-β expression by ATRA in MRC5 fibroblasts.
Conclusion: Pulmonary emphysema fibroblasts fail to express elastin under ATRA stimulation. CRABP2, which is necessary for elastin induction by ATRA in MRC-5 cells, is expressed at low levels in emphysema fibroblasts. This alteration in the retinoic acid signalling pathway in lung fibroblasts may contribute to the defect of alveolar repair in human pulmonary emphysema. These results are the first demonstration of the involvement of CRABP2 in elastin expression.
Statistics from Altmetric.com
Pulmonary emphysema is a chronic degenerative lung disease characterised by an imbalance between alveolar destruction and repair which results in the progressive destruction of pulmonary alveoli and chronic respiratory failure. Lung fibroblasts and myofibroblasts play a major role in the course of pulmonary repair processes,1 notably through the secretion of elastin, an essential component of the pulmonary extracellular matrix.2
Signalling by retinoic acid, the main active metabolite of vitamin A, is of particular importance for the development, maintenance and repair of pulmonary alveoli, as assessed by the following arguments: firstly, elevation of retinoic acid levels in the lung is a stimulus for the alveologenesis phase of lung development3; secondly, all-trans retinoic acid (ATRA) induces the expression of elastin in lung fibroblasts4; thirdly, vitamin A deficiency leads to an emphysema-like phenotype in the lung of adult rats5; finally, the systemic administration of ATRA has been reported to abrogate elastase induced emphysema in adult rats and mice.6 7 Retinoic acid exerts its effects by binding two families of nuclear receptors, the retinoic acid receptors (RAR-α, β and γ) and the retinoid X receptors (RXR-α,β and γ), which translocate to the nucleus on binding where they act as transcription factors.8 The binding of retinoic acid to RARs and the transcriptional activity of RARs are greatly enhanced by a 15 kDa cytosolic protein, cellular retinoic acid binding protein 2 (CRABP2).9–11
In light of those elements, we hypothesised that an alteration in the retinoic acid signalling pathway might contribute to the defect of alveolar repair that is observed in human pulmonary emphysema. To explore this hypothesis, we focused on elastin production by lung fibroblasts. We first determined whether ATRA induced elastin and RAR-β mRNA expression in lung fibroblasts cultured ex vivo from human control and emphysematous lung samples. Then, expression of RAR-α, RAR-β, RAR-γ, RXR-α, RXR-β, RXR-γ, CRABP1 and CRABP2 was determined in those cells. As we found a selective reduction in CRABP2 expression in fibroblasts from emphysematous patients, we determined whether suppression of CRABP2 expression in lung fibroblasts using a siRNA strategy abolished the induction of elastin expression by retinoic acid.
MATERIALS AND METHODS
Lung samples
The study was approved by the ethics committee of Paris-Bichat University Hospital, Paris, France. Patients gave informed consent.
Emphysema patients
Fibroblasts were cultured from lung samples from eight patients with severe pulmonary emphysema undergoing lung volume reduction surgery (n = 3) or lung transplantation (n = 5). Median age of the patients was 58 years (interquartile range (IQR) 53, 58.5). All patients were smokers or ex-smokers (33 pack-years, IQR 30, 38) and had normal plasma α1 antitrypsin levels. Emphysema was diagnosed in the presence of an obstructive ventilatory disorder and overdistension on lung function tests associated with characteristic chest CT and histological findings, and the absence of any associated lung disease was verified. The median total lung capacity of patients with emphysema was 127% predicted (IQR 122, 129) and median forced expiratory volume in 1 s 28% predicted (IQR 18, 35).
Control patients
Fibroblasts were cultured from lung samples from 10 patients undergoing lung surgery for cancer. The age of the control subjects (68 years, IQR 65, 71) was not different from that of patients with emphysema (p = 0.13). Lung samples were taken from an uninvolved segment, and the absence of emphysema was verified microscopically. Five patients were active or past smokers (36 pack-years, IQR 30, 40) and five were never-smokers. The median total lung capacity of the control patients was 98% predicted (IQR 86, 114) and their median forced expiratory volume in 1 s was 90% predicted (IQR 75, 107).
Isolation of pulmonary fibroblasts
Pulmonary fibroblasts were cultured from lung explants, as previously described.12 Fibroblasts were cultured with DMEM culture medium (Gibco/Invitrogen, Cergy-Pontoise, France) with 10% fetal calf serum (Fetalclone 2; Hyclone, Logan, Utah, USA), 100 UI/ml penicillin G, 100 μg/ml streptomycin sulphate and 0.25 μg/ml amphotericin B (Gibco/Invitrogen). Cells were maintained at 37°C with 5% CO2, and were used at passage 5. To confirm the fibroblastic nature of cultured cells, all cell cultures were evaluated immunocytochemically at passage 5. All cells stained positive with anti-vimentin, anti-desmin and anti-prolyl-4-hydroxylase antibodies (DakoCytomation, Trappes, France). Three of the 10 control cultures and all emphysema cultures contained 5–10% of α smooth muscle actin positive cells. Staining with antibodies directed against smooth muscle myosin heavy chain-1, pancytokeratin and CD31 was always negative.12
Modulation of elastin and RAR-β mRNA expression by retinoic acid in pulmonary fibroblasts
Pulmonary fibroblasts (100 000/well) were seeded in 6 well plates and cultured to 80% confluence. They were then incubated with incremental concentrations of ATRA (10−9 M to 10−5 M) for 72 h. ATRA was dissolved in dimethylsulfoxide (DMSO), and cells cultured with 1% DMSO were used as controls. Total RNA was extracted with the Nucleospin extraction system (Macherey-Nagel, Hoerdt, France) and reverse transcribed into complementary DNAs (cDNAs) with MMLV retro transcriptase (Invitrogen) according to the manufacturer’s instructions. Quantitative real time PCR using a SybrGreen fluorochrome (Sigma, St Quentin-Fallavier, France) was performed with a Mx3000P thermocycler (Stratagene, La Jolla, California, USA) to quantify elastin and RAR-β cDNAs as well as ubiquitin C (UBC) cDNA as an endogenous control.13 cDNA copy numbers were expressed relative to a standard prepared from pooled lung fibroblast cDNAs that were used for all experiments. Amplification specificity was verified by agarose gel electrophoresis and melting curves.
Determination of the intracellular content of fibroblasts in RAR, RXR, CRABP1 and CRABP2 mRNA in the absence of stimulation
RAR-α, RAR-β, RAR-γ, RXR-α, RXR-β, RXR-γ, CRABP1 and CRABP2 mRNAs were quantified by reverse transcription-PCR in unstimulated control and emphysema fibroblasts, as described above. Primers sequences are listed in table 1.
Determination of the intracellular content of CRABP2 protein in fibroblasts
Fibroblasts at passage 5 were cultured to confluence in 75 cm2 flasks (Corning, Schiphol-Rijk, The Netherlands). Cells were rinsed twice with phosphate buffered saline (Gibco/Invitrogen) and proteins were extracted with Cytobuster Protein Extraction Reagent (Novagen, Madison, USA) according to the manufacturer’s protocol. Cellular proteins (25 μg) were separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis, transferred onto a PVDF membrane (Biorad, Marne-la-coquette, France) and incubated with a mouse monoclonal primary antibody binding CRABP2 (5CRA-3B3)14 diluted 1/1000 for 1 h at room temperature. A mouse monoclonal antibody binding β-actin (A-5316; Sigma) was used as an endogenous control. Detection was performed with peroxidase conjugated sheep anti-mouse (NA 931) and donkey anti-rabbit (NA934) antibodies and ECL reagent (Amersham, Little Chalfont, UK). The Bio-Vision system (Fisher Bioblock Scientific, Illkirch, France) was used for densitometric quantification of protein bands.
Modulation of elastin and RAR-β mRNA expression by retinoic acid in MRC-5 fibroblasts treated with a CRABP2 siRNA
In preliminary experiments, a decrease in the cellular content in CRABP2 protein was obtained only if cells were transfected at the time of seeding and grown in the continued presence of the siRNA and transfection reagents. As primary lung fibroblasts failed to grow in those conditions, the MRC-5 human lung fibroblast cell line (Eurobio, Les Ulis, France) was used to determine the role of CRABP2 in ATRA induced elastin expression.
MRC-5 cells were seeded at a density of 100 000 cells/well in 6 well culture plates. The same day, these cells were transfected with either 20 nM of anti-CRABP2 siRNA (sense: 5′-GCG CAC CAC AGA GAT TAA CTT CAA G-3′) or 20 nM scramble control RNA (sense 5′-GCG CAC CGA GAA TTA TTC ACA CAA G-3′; Stealth Technology, Invitrogen) using Lipofectamine 2000 (Invitrogen), according to the manufacturer’s protocol, and were incubated with 10% fetal bovine serum. Fresh medium, fetal bovine serum, RNAs and Lipofectamine 2000 were added every 3 days until cellular subconfluence was obtained. Transfected cells were then treated with incremental concentrations of ATRA, and elastin, RAR-β and UBC mRNAs were quantified as described above. The CRABP2 mRNA and protein content in siRNA and control RNA treated cells was determined by RT-PCR and western blotting, as described above.
Statistical analysis
Data are expressed as median and interquartile range (IQR), expressed as the values for the 25th and 75th centiles. Differences between control and emphysema fibroblasts were determined using the Mann–Whitney U test. To compare the effect of retinoic acid on baseline conditions, we used the Friedman analysis of variance test, followed by Wilcoxon’s paired test for group comparisons if a difference was detected. A p value <0.05 was considered significant. In the figures, data are presented as box plots showing the median value, 25th and 75th centiles, and extreme values.
RESULTS
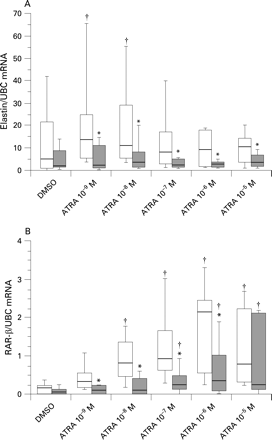
ATRA increased the elastin mRNA content of control but not emphysema fibroblasts
Elastin mRNA was detected in all cell cultures studied except two control fibroblast cultures. In the absence of stimulation, the elastin/UBC mRNA content of emphysema fibroblasts (1.97, IQR 1.21, 6.17) was not different from that of control fibroblasts (4.8, IQR 1.04, 19.8; p = 0.47).
In control fibroblasts, treatment with 10−9 M and 10−8 M ATRA induced 182% (p = 0.01) and 126% (p = 0.028) increases in the median elastin/UBC mRNA ratio, respectively. In emphysema fibroblasts, the elastin/UBC mRNA ratio was not modified by any concentration of ATRA (fig 1A). In the presence of ATRA at any concentration, the elastin/UBC mRNA ratio was superior in control compared with emphysema fibroblasts.
ATRA increased the RAR-β mRNA content of control and emphysema fibroblasts
In order to determine whether the absence of elastin mRNA induction by ATRA in emphysema fibroblasts was specific for the mechanisms governing ATRA induced elastin expression or was a result of a general deficiency in the retinoic acid signalling pathway, expression of RAR-β, which is induced by ATRA in fibroblasts,15 was determined in control and emphysema fibroblasts in the presence of ATRA.
RAR-β mRNA was detected in all but one control and one emphysema culture. In the absence of stimulation, the RAR-β mRNA content of emphysema fibroblasts was not different from that of control fibroblasts.
In control fibroblasts, ATRA at a concentration of 10−8 M and above induced an increase in the RAR-β mRNA content, reaching a maximum at 10−6 M with a median 13.5-fold increase (p = 0.005). In emphysema fibroblasts, though this effect was inferior to that observed in control fibroblasts, induction of RAR-β mRNA expression was also observed with ATRA at a concentration of 10−7 M and above, with a median 5.2-fold increase at 10−6 M (p = 0.03).
Low expression of CRABP2 mRNA and CRABP2 protein in emphysema fibroblasts
RAR-α, RAR-β, RAR-γ, RXR-α, RXR-β, RXR-γ and CRABP2 mRNAs were detected in all fibroblast lines. CRABP1 mRNA was not detected in these cells. The intracellular content in RAR-α, -β and -γ and RXR-α, -β and -γ mRNA was not different in control and emphysema fibroblasts, while the median intracellular content of control fibroblasts in CRABP2 mRNA was eight-fold superior to that of emphysema fibroblasts (fig 2A).
We determined by western blotting whether the inferior content in CRABP2 mRNA translated to inferior content in CRABP2 protein in emphysema fibroblasts compared with control fibroblasts. Median intracellular content of CRABP2 in emphysema fibroblasts in relation to that of β-actin (0.61, IQR 0.58, 1.74) was lower than that of control fibroblasts (0.915, IQR 0.14, 0.79, p = 0.05) (fig 2B, C).
ATRA failed to induce elastin mRNA expression in MRC-5 fibroblasts treated with a siRNA targeting CRABP2 mRNA while RAR-β induction was preserved
To determine whether the low expression of CRABP2 could explain the absence of elastin induction by ATRA, we tested whether CRABP2 gene silencing affected elastin and RAR-β induction by ATRA in the MRC-5 pulmonary fibroblast cell line.
Median CRABP2 mRNA was reduced by 97.6% in cells treated with the anti-CRABP2 siRNA compared with cells treated with the control RNA (p = 0.045, fig 3A). Median content in CRABP2 protein of siRNA treated cells was reduced by 95.5% compared with that of control RNA treated cells (p = 0.045, fig 3B). The anti-CRABP2 siRNA had no additional cytotoxicity compared with the control RNA.
In MRC-5 fibroblasts treated with the control RNA, exposure to ATRA at all concentrations induced an increase in the median elastin/UBC mRNA ratio which reached a maximum of 511% at an ATRA concentration of 10−7 M. Induction of elastin gene expression by ATRA was completely abrogated in cells treated with the anti-CRABP2 siRNA (fig 3C). In the presence of ATRA at any concentration, the elastin/UBC mRNA ratio was superior in MRC-5 cells treated with the control RNA compared with cells treated with the anti-CRABP2 siRNA.
In contrast, CRABP2 silencing did not abrogate the induction of RAR-β mRNA by ATRA in those cells. In siRNA as well as in control RNA treated cells, ATRA at a concentration of 10−7 M and above induced an elevation in the RAR-β mRNA content of cells (fig 3D).
DISCUSSION
The main results of this study are as follows: (1) retinoic acid induced elastin mRNA expression in control but not in emphysema lung fibroblasts while it induced RAR-β mRNA in both groups, (2) this discrepancy was associated with decreased levels of CRABP2 mRNA and protein in emphysema fibroblasts and (3) silencing of CRABP2 expression in the MRC5 human lung fibroblast cell line reproduced the phenotype of emphysema fibroblasts, as it resulted in the loss of elastin mRNA but not RAR-β induction by retinoic acid. These results are the first demonstration of the involvement of CRABP2 in elastin expression and strongly suggest that the lack of elastin induction by retinoic acid in emphysema fibroblasts is related to the low expression of CRABP2 in those cells.
In our experiments, ex vivo culturing of lung fibroblasts implicated selection bias towards cells able to proliferate outside of their normal environment. Moreover, whether the reduction in CRABP2 expression by cultured emphysema fibroblasts was related to a global reduction of its expression in lung fibroblasts in this disease or reflected the loss of a particular subgroup of CRABP2 expressing lung fibroblasts cannot be determined from our study. Interestingly, skin fibroblasts express different levels of CRABP2 depending on the site of their isolation, as subcutaneous fat fibroblasts express much lower levels of CRABP2 than their dermis derived counterparts.16 Regulation of CRABP2 expression is incompletely understood but seems to involve the RARs. However, alteration of this pathway seems unlikely in the present study as expression of RAR mRNAs was similar in fibroblasts from emphysematous and control patients. The AP-2 transcription factor17 and protein kinase C18 have also been involved in the regulation of CRABP2 expression, but their role in pulmonary emphysema is unknown. Whatever the mechanism of the reduced CRABP2 expression in fibroblasts from patients with emphysema, the present results are in line with previous reports showing that the phenotype of lung fibroblasts is deeply altered in the emphysematous lung, as those cells have been shown to express markers of cellular senescence,19 to have a reduced proliferation rate20 and to secrete low amounts of hepatocyte growth factor, a key mitogen for alveolar epithelial cells,12 while they fail to link the endothelial and epithelial compartments of the lung through direct intercellular contacts, as is observed in the normal lung.21
In our experiments, ATRA increased the elastin mRNA content in fibroblasts in a non-dose dependent manner as the maximal effect was observed at ATRA concentrations of 10−9 M in primary cells and 10−7 M in MRC-5 cells. We do not know the reason for this lack of a dose dependent effect. However, a similar phenomenon was described in human primary skin fibroblasts in which ATRA potentiated 8-bromo-cAMP induced hepatocyte growth factor. In those cells, the maximal effect of ATRA was obtained at a concentration of 10−7 M.22
It may be argued that the different RAR-β mRNA expression that was observed between control and emphysema fibroblasts in the presence of 10−8 M to 10−6 M ATRA may have contributed to the different response of those cells to ATRA with regard to elastin expression. However, this hypothesis seems unlikely as RAR-β has been consistently shown to inhibit alveolar septation, of which elastin synthesis is an essential step.23 24 Moreover, in addition to the silencing experiments performed in MRC-5 cells, demonstration that CRABP2 deficiency accounted for the reduced elastin synthesis in emphysema fibroblasts would have been strengthened by the transfection of those cells with an expression vector that increases CRABP2 to the level of controls. This experiment could not be performed in this study as primary cells could not survive in the presence of the transfection reagents.
Elastic fibres, an essential component of the lung extracellular matrix as they provide the elasticity needed for cyclic ventilation, are extremely stable and undergo very limited renewal and remodelling in healthy individuals.25 Their degradation is a hallmark of human emphysema26 as well as of numerous animal models of this disease.27 28 While elastic fibre repair has not been demonstrated to date in the human emphysematous lung, data obtained from animal studies point towards a potentially essential role of the lack of elastic fibre regeneration and repair in the constitution of emphysema lesions. Treatment of mice with beta-aminopropionitrile, a chemical inhibitor of elastin cross linking, leads to aggravated emphysema after elastase instillation,29 while mice expressing low levels of elastin develop more severe emphysema than wild-type mice after exposure to cigarette smoke.30 Thus from a pathophysiological point of view, demonstration that fibroblasts cultured from human emphysematous lung have an impaired capacity to express elastin mRNA under retinoic acid stimulation may help explain the defect in alveolar maintenance and repair in this disease. It must be noted that our study was limited to the exploration of elastin mRNA and that differences in the post-transcriptional regulation of elastin production may have been overlooked.
Given the beneficial effect of ATRA in animal models of emphysema, the therapeutic potential of this molecule was evaluated in human patients with emphysema. Disappointingly, a 3 month course of ATRA could not reverse the functional and morphological changes associated with emphysema, despite obtaining high plasma ATRA levels.31 Our results may provide an explanation for the lack of a therapeutic effect of ATRA in humans with emphysema as CRABP2, which is necessary for induction of elastin expression by retinoic acid, is lacking in pulmonary fibroblasts in those patients. Interestingly, ATRA induced alveologenesis seems to be strain dependent in mice,32 and it may be hypothesised that differences in CRABP2 expression in the lung between strains may contribute to this phenomenon. Whether synthetic CRABP2 independent retinoids would have a beneficial effect in pulmonary emphysema remains to be investigated.
Overall, our results indicate that low expression of CRABP2 is associated with loss of elastin gene expression by retinoic acid in fibroblasts cultured from human emphysematous lung, which may participate in the lack of alveolar repair and may help explain the lack of a therapeutic effect of ATRA in this disease.
Acknowledgments
We thank Dr Nadira Houhou (Service de Virologie, Hôpital Bichat) for kindly providing the MRC-5 cells used in this study.
REFERENCES
Footnotes
Funding: LP was supported by a research fellowship from INSERM (Poste d’Accueil) and a grant from the Société de Pneumologie de Langue Française (SPLF). JB was supported by INSERM and Assistance Publique-Hôpitaux de Paris (Contrat d’Interface). Part of this project was supported by a grant from the Chancellerie des Universités (Legs Poix).
Competing interests: None.
Ethics approval: The study was approved by the ethics committee of Paris-Bichat University Hospital, Paris, France.